 |
By Jamshed Arslan Pharm.D.
Multiple myeloma (MM) is a cancer of antibody-producing plasma cells. The bone marrow (BM) of MM patients is hypoxic, and MM cells overexpress many cancerous genes that are regulated by hypoxia-inducible factors (HIFs). Cancer stem cells (CSCs) in the hypoxic BM regions are blamed for the incurability of MM, because CSCs are often resistant to drugs currently used against BM cancers (including proteasome inhibitors and immunomodulatory agents). Dr. Eishi Ashihara at the Kyoto Pharmaceutical University, Japan, and colleagues, set out to characterize the biology of MM stem cells. They found that TGF-beta/Smad pathway activation is responsible for the stemness (augmented self-renewal, enhanced mRNA levels of stem cell markers, and increased tumorigenicity) of hypoxia-adapted MM (HA-MM) cells.
Researchers transplanted human myeloma cell lines (parental MM cells) into the epiphysis of immunocompromised mice, which led to their death within a month. When transplanted, mice were injected with a hypoxia marker (pimonidazole) and immunohistochemical staining revealed that MM cells in the BM were hypoxic, just like canonical CSCs. To confirm this, parental MM cells were kept in low oxygen, and HA-MM cells were selected based on their ability to grow for more than 6 months in hypoxia. Western blotting showed an upregulation of HIF-1alpha in HA-MM cells. The mRNA expression of stem cell markers (NANOG, OCT4, SOX2) was also higher in HA-MM cells than in the parental MM cells. When colony growth was evaluated by clonogenic replating assays, HA-MM cells had higher replating efficiency and number of cells per colony, as compared to parental MM cells. Furthermore, a slower growth of HA-MM cells as compared to parental cells confirmed their CSC-like phenotype.
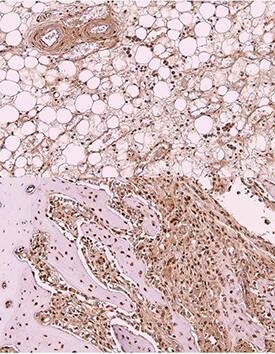 Immunohistochemistry-Paraffin: OCT4 Antibody (486) [NBP2-15052] - Imaging of Rabbit stem cells in Bone marrow of tibia. This image was submitted via customer Review. *{Rabbit IHC-P}*
Immunohistochemistry-Paraffin: OCT4 Antibody (486) [NBP2-15052] - Imaging of Rabbit stem cells in Bone marrow of tibia. This image was submitted via customer Review. *{Rabbit IHC-P}*
The stem cell phenotype of HA-MM cells was then clarified in vivo. The lifespan of immunodeficient mice injected with HA-MM cells was nearly halved when compared to those injected with parental MM cells. Researchers harvested the BM of transplanted mice, and MM cells positive for the plasma-cell marker (CD138) were then injected to other immunodeficient mice. As expected, all mice injected with HA-MM cells died, whereas most animals injected with parental MM cells survived. The decrease in mice survival further validated the stem cell-like pathogenic characteristics of HA-MM cells.
Western blotting showed that HA-MM cells have significantly higher levels of phosphorylated Smad2 (but not beta-catenin) than did the parental MM cells. This indicated that hematopoiesis-regulating TGF-beta/Smad signaling (but not Wnt/ beta-catenin pathway) is involved in maintaining stemness in hypoxia. To validate the findings, a TGF-beta/Smad inhibitor was used, and flow cytometry revealed that the inhibitor decreased HA-MM cells in G0 phase (quiescent cells), but increased the S/G2/M fractions. TGF-beta/Smad inhibition also decreased the replating efficiency of HA-MM cells. This means that TGF-beta/Smad pathway takes part in maintaining stemness and clonogenicity of HA-MM cells.
These findings suggest that HIF-1 may directly activate Smad signaling, thereby stimulating the study of interactions between HIFs and TGF-beta/Smad. Moreover, this investigation supports therapies targeting TGF-beta/Smad pathway for the treatment of MM and other CSC-related pathologies.
Request TGF-beta Pathway Poster
 Jamshed Arslan, Pharm D.
Jamshed Arslan, Pharm D.
University of Alabama at Birmingham, School of Medicine
Dr. Arslan studies cell signaling in mitochondrial defects in C. elegans and transgenic mice.
References
Nakagawa, Yoko, et al. “Multiple Myeloma Cells Adapted to Long-Exposure of Hypoxia Exhibit Stem Cell Characters with TGF-beta/Smad Pathway Activation.” Biochemical and Biophysical Research Communications,2018, n. pag. doi: 10.1016/j.bbrc.2018.01.034.