 |
By Yoskaly Lazo-Fernandez, PhD
The first description of what is called today an autophagosome was given in a paper published in 1957. Its author employed electron microscopy to observe the neonatal features of mouse kidneys1. Autophagosomes where then described as large round bodies found in the cytoplasm, primarily of proximal tubule epithelial cells, and consisting of an amorphous material containing concentrically lamellar structures and mitochondria. It was not by chance that these structures were found in kidney tubules, because autophagy plays an essential role in kidney function both in health and disease2,3. This blog will briefly introduce the main pathophysiological areas of connection between autophagy and the kidneys. Future blogs will expand on these topics.
Autophagy is a protective mechanism that results in the degradation of dangerous protein aggregates, infectious agents, and defective organelles, which prevents inflammation, oxidative stress and apoptosis. Consequently, defects in autophagy in the kidneys, as well as in other organs, lead to cellular injury. Specific kidney conditions that have been associated with autophagy defects include acute kidney injury4, chronic kidney disease5, renal fibrosis6, glomerular disease7, renal autoimmune and inflammatory responses8, polycystic kidney disease9, diabetic nephropathy10 and metabolic acidosis11.
In general, conditions that affect nutrient or oxygen levels in kidney cells will induce a strong protective autophagy response. For example, hypoxia is a dangerous condition particularly common in the kidneys and strongly correlated with both acute kidney injury and with end stage chronic kidney diseases. Ischemic insults induce apoptosis or necrosis in kidney epithelial cells, as well as kidney vasoconstriction, inflammation and fibrosis. Low oxygen levels cause dissociation of the key autophagy inhibitor mTORC1 from its target ULK112, independently from energy-signaling, thus allowing ULK1 to activate autophagy as a protective mechanism.
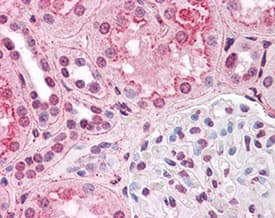 Immunohistochemistry: HIF-1 alpha Antibody (H1alpha67) [NB100-105] - Staining of HIF1 alpha in human kidney. Renal tubular epithelium showed moderate membranous, cytoplasmic and nuclear staining, and glomeruli showed faint to moderate nuclear staining.
Immunohistochemistry: HIF-1 alpha Antibody (H1alpha67) [NB100-105] - Staining of HIF1 alpha in human kidney. Renal tubular epithelium showed moderate membranous, cytoplasmic and nuclear staining, and glomeruli showed faint to moderate nuclear staining.
The The other most common connection between autophagy and kidney diseases is the deregulation of nutrient-sensing signals, usually observed during aging and in diabetes- or obesity-mediated kidney changes. In these cases, prolonged high intracellular levels of glucose cause stimulation of mTORC1’s activity leading to chronic inhibition of autophagy. Kidney injury then follows from the accumulation of the damage caused by reactive oxygen species (ROS) and protein aggregates, particularly of advanced glycation end products13.
Autophagy induction through the inhibition of mTORC1 signaling, both pharmacologically and by calorie restriction, has thus been reported as beneficial in numerous models of kidney disease14–16. Other disease- and cell-specific autophagy related targets are being actively studied in the search of new therapies, offering new hope for the more than 15% of adults in the United States that suffer from kidney diseases.
 Yoskaly Lazo Fernandez, PhD
Yoskaly Lazo Fernandez, PhD
Emory University, Department of Medicine/Renal Division
Dr. Lazo-Fernandez is interested in understanding the dietary factors that contribute to the development of hypertension and other chronic diseases.
References