 |
By Jamshed Arslan Pharm.D.
Interneurons transmit impulses between other neurons, in part, to facilitate the birth of neurons. Cortical interneurons themselves arise from the progenitors in the ventral telencephalon, a brain region that generates basal ganglia. The role of mechanistic target of rapamycin (mTOR) signaling in this process is poorly understood even though mTOR is known to determine brain size. By deleting mTOR in mouse interneuron progenitors and their progeny, Dr. Woo-Yang Kim’s team at the University of Nebraska Medical Center, USA found two homeostatic activities of mTOR in the developing brain: regulation of autophagy and quantity of cortical interneurons.1
The team’s first step in evaluating the role of mTOR in interneuron development was to perform cre-lox-mediated mTOR deletion in the GABAergic interneuron progenitors in a fetal brain region called ganglionic eminence (GE). DAPI staining revealed a reduced size, thickness, and number of cells in the GE of ventral telencephalon. As compared to the controls (mTORloxP/+; Tg(mI56i-cre,EGFP)1Kc/Dlx5/6-CIE), the number of GFP-positive interneurons in the mutant (mTORloxP/loxP; Tg(mI56i-cre, EGFP)1Kc/Dlx5/6-CIE) brain was decreased by 29%, 30%, and 32% in the lateral, medial, and dorsal cerebral cortices, respectively. Immunostaining with cell proliferation markers – BrdU, MKI67/KI67, and most importantly, phospho-H3f3 (with/without rapamycin treatment) – confirmed the mTOR-deletion-induced suppression of GE interneuron progenitors.
“mTOR plays an important role in determining ventral brain size via regulating autophagy and proliferation of inhibitory progenitors.”
Woo-Yang Kim, PhD.
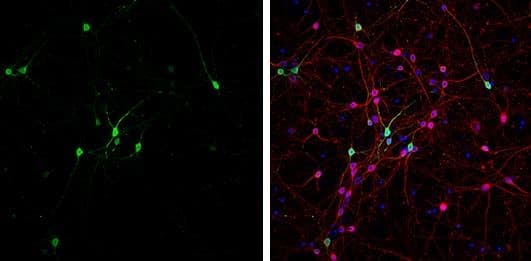
Immunocytochemistry/Immunofluorescence: GABA Antibody [NBP2-43558] - DIV9 rat E18 primary cortical neurons were fixed in 4% paraformaldehyde at RT for 15 minutes. Green: GABA stained by GABA antibody diluted at 1:500. Red: beta Tubulin 3/ Tuj1, stained by beta Tubulin 3/ Tuj1 antibody [MAB1195-SP] diluted at 1:500. Blue: Fluoroshield with DAPI.
Perhaps the most intriguing aspect of their findings was that the ratios of dorsal-to-medial and medial-to-lateral interneurons, or the percentage of interneurons in different cortical layers, and the length and width of leading processes of cortical interneurons in the knockouts were similar to those in the controls. In other words, mTOR deletion reduced the number, but not the proportional positioning or morphology, of the cortical interneurons.
"mTOR determines overall brain size as we published [previously].2 The mTOR-mediated regulation on brain size influences not just inhibitory neurons, but excitatory cortical neurons. In [this] paper,1 however, we selectively knocked out mTOR in inhibitory neurons, which reduced ventral brain size. The reduction in cortical interneurons is probably less influential in determining cortex size because ~20% of cortical neurons are interneurons.” Woo-Yang Kim, PhD.
Kim’s team is the first to establish an association between the mTOR pathway and autophagy in the developing GE. Western blotting indicated an increase in the autophagy-associated proteins (BECN1/Beclin 1; MAP1LC3B/LC3B; and SQSTM1/p62) in the GE lysate of knockout mice (mTORloxP/loxP; Nkx2–1-Cre) as compared to the controls (mTORloxP/+; Nkx2.1-Cre). After discovering a reduced level of phospho-RPS6 in GE lysates, which is a sign of autophagy, they cultured and immunostained the progenitor cells with LC3 (a central protein in the autophagy pathway) and mTOR antibodies. As expected, only the mTOR-deficient cells had LC3 aggregates, indicating that mTOR-loss induces autophagy.
To determine the functional relevance of these results, cultured GE progenitors were treated with FOXO-inhibitors, with and without rapamycin, to suppress autophagy. MKI67 and phospho-H3f3 staining showed rapamycin-induced decrease in proliferating progenitors. Inhibiting FOXO rescued proliferation by reversing the rapamycin-caused mTOR-inhibition. These findings indicated that increased autophagy by mTOR inhibition stifles interneuron development.
Order Your Autophagy Handbook Today!
The findings are important in that they provide a path for the discovery of treatments for autism, epilepsy, intellectual disability and other neurodevelopmental issues with abnormal number of cortical interneurons. The team has found a novel function of mTOR for interneuron proliferation and autophagy in a developing mammalian brain and suggest an mTOR-independent mechanism of neuronal migration. Overall, the researchers have placed mTOR-related neural plasticity and connectivity at the heart of therapeutic strategies against psychiatric and neurodevelopmental disorders.
Explore New Autophagy Research Area
 Jamshed Arslan, Pharm D.
Jamshed Arslan, Pharm D.
University of Alabama at Birmingham, School of Medicine
Dr. Arslan studies cell signaling in mitochondrial defects in
C. elegans and transgenic mice.
References