 |
By Jamshed Arslan Pharm.D., PhD.
Kinome describes kinases, and kinomics refers to the kinase signaling. Studying the effects of reagent (exogenously applied growth factor or inhibitor) on kinase activity is a common experiment in cell, developmental and integrative biology laboratories. This blog offers a cost-effective and robust method of obtaining adequate amount of sample for kinomic analysis from mammalian culture cells like HEK cells after treatment with reagent. Using this method, you can easily get over 50 μg protein sample in 50 μl volume from a confluent well of a standard 6-well plate with 0.5 x 106 cells. This concentration (1 μg/μl) is enough for kinomic analysis, for example, through phospho-receptor tyrosine kinase array kits or serine/threonine kinome chips.
Explore Phospho Specific Antibodies
![AKT1 and AKT1 [p Ser473] in mouse 3T3 cell lysates following PDGF treatment, WB](https://images.novusbio.com/design/AKT1-p-Ser473.png)
|
Independent Antibodies Validation and Biological Strategies Validation. Western Blot: AKT1 [p Ser473] Antibody (104A282) [NB100-56749] - Total protein from mouse 3T3 cells treated with and without PDGF (50 ng/ml) for the indicated times was separated on a 7.5% gel by SDS-PAGE, transferred to PVDF membrane and blocked in 5% non-fat milk in TBST. The membrane was probed with 2.0 ug/ml anti-AKT1 (NBP2-01725) and 2 ug/ml pS473 AKT1 in 1% BSA in TBST and detected with an anti-mouse HRP secondary antibody using chemiluminescence. Note the detection of phosphorylated AKT1 in response to PDGF treatment compared to total AKT1 protein. |
|
ERK1 (T202/Y204)/ERK2 (T185/Y187) 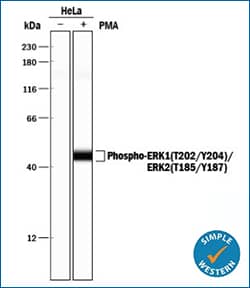
|
Simple Western lane view shows lysates of HeLa human cervical epithelial carcinoma cell line untreated (-) or treated (+) with PMA, loaded at 0.2 mg/mL. A specific band was detected for ERK1 (T202/Y204)/ERK2 (T185/Y187) at approximately 44 kDa (as indicated) using 5 μg/mL of Rabbit Anti-Human/Mouse/Rat Phospho-ERK1 (T202/Y204)/ERK2 (T185/Y187) Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1018). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system. |
![FANCD2 [p Ser222] in irradiated lymphoblasts, WB](https://images.novusbio.com/design/FANCD2-p-Ser222.png)
|
Biological Strategies Validation and Genetic Strategies Validation. Western Blot: FANCD2 [p Ser222] Antibody [NB100-502] - Indicated lymphoblasts (PD7, WT: GM1526, AT) were irradiated with 15 Gy (2), and immunoblotted with anti-FANCD2 and anti-FANCD2 (pS-222) |
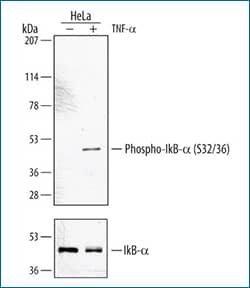
|
Western blot shows lysates of HeLa carcinoma cell line untreated (-) or treated (+) with 20 ng/mL Recombinant Human TNF-alpha (Catalog # 210-TA) for 5 minutes. PVDF membrane was probed with 1 μg/mL Rabbit Anti-Human Phospho-IkB-alpha (S32/S36) Antigen Affinity-purified Polyclonal Antibody (Catalog # AF4809) followed by HRP-conjugated Anti-Rabbit IgG Secondary Antibody (Catalog # HAF008). A specific band for Phospho-IkB-alpha (S32/S36) was detected at approximately 44 kDa (as indicated). For additional reference, duplicate lysates were probed with 0.1 μg/mL Human IkB-alpha Monoclonal Antibody (lower panel, Catalog # MAB4299). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1. |
Typically, a tyrosine kinase chip with 4 arrays (i.e. 4 samples) is cheaper than the serine/threonine kinome chip. To save bucks, you can pool the contents of triplicate, and submit two samples (reagent-treated vs. control) per 6-well plate for kinomic analysis. Using R&D Systems multiplex array kits, you can cost-effectively detect multiple phosphorylated proteins in a single sample.
All in all, Kinase activity plays a major role in cell signaling. Sample collection using the above-mentioned techniques can help optimize your kinomic assays and facilitate the identification of signaling mechanisms important in selecting potential lead compounds for drug development.
View Relevant Signaling Pathways
 Jamshed Arslan, Pharm D., PhD.
Jamshed Arslan, Pharm D., PhD.
University of Alabama at Birmingham, School of Medicine
Dr. Arslan studies cell signaling in mitochondrial defects in
C. elegans and transgenic mice.
References
Kilpinen, Sami, Kalle Ojala, and Olli Kallioniemi. "Analysis of Kinase Gene Expression Patterns across 5681 Human Tissue Samples Reveals Functional Genomic Taxonomy of the Kinome." PLoS ONE, vol. 5, no. 12, 2010, n.pag. https://doi.org/10.1371/journal.pone.0015068
Silva, Richard C., Beatriz A. Castilho, and Evelyn Sattlegger. "A Rapid Extraction Method for Mammalian Cell Cultures, Suitable for Quantitative Immunoblotting Analysis of Proteins, Including Phosphorylated GCN2 and eIF2α." Methods X, vol. 5, 2018, pp. 75-82. https://doi.org/10.1016/j.mex.2017.10.008