 |
By Beth Melson, MS
Antibiotic resistance is a global threat to public health. Widespread, inappropriate use of antibiotics, such as to treat viral infections or promote growth in livestock, has led to increased incidence of antibiotic resistance1. Resistance is costly to both human health as well as the economy1, and thus the identification of new, effective antibiotics is a public health priority. One solution to this problem is to repurpose commercially available, FDA-approved drugs to target host cell processes that would limit bacterial proliferation2. The benefits of using repurposed drugs include established low toxicity to human cells, and the reduced likelihood that pathogens will develop resistance to these drugs, as the pathogen is not directly targeted3.
Staphylococcus aureus is a bacterial pathogen that is asymptomatically carried in the nasal passages by approximately 40% of people3. According to the CDC, methicillin-resistant S. aureus, or MRSA infection, is a serious threat due to its extensive drug resistance. S. aureus causes severe and life-threatening illness, partly due to its ability to survive inside host cells, escaping detection by the immune system and limiting exposure to antibiotics3. The development of novel anti-infective approaches is critical to combat this important pathogen.
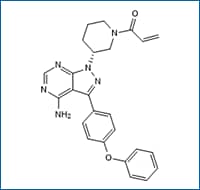 Ibrutinib Cat. No. 6813, Potent and selective BTK inhibitor (IC50= 0.5 nM). Selective for BTK against a screening panel of kinase enzymes. Inhibits autophosphorylation of BTK, phosphorylation of PLCγ, and phosphorylation of ERK (IC50 of 11 nM, 29 nM and 13 nM, respectively).
Ibrutinib Cat. No. 6813, Potent and selective BTK inhibitor (IC50= 0.5 nM). Selective for BTK against a screening panel of kinase enzymes. Inhibits autophosphorylation of BTK, phosphorylation of PLCγ, and phosphorylation of ERK (IC50 of 11 nM, 29 nM and 13 nM, respectively).
Using a high-throughput screen of 133 host-targeted FDA-approved drugs against MRSA-infected host cells, three drugs were identified that reduced intracellular MRSA with minor effects on host cell viability: Ibrutinib, Dasatinib, and Crizotinib3. Ibrutinib was selected for further investigation due to its inhibition of MRSA survival and high reproducibility. Importantly, the inhibitory effect of Ibrutinib was due to direct effects on the host cells, as the drug did not affect growth of MRSA in pure culture. Treatment of MRSA-infected host cells with Ibrutinib increased cell viability compared to untreated infected cells. The largest effects on intracellular MRSA were found early after infection, suggesting that Ibrutinib affects host cell internalization of MRSA.
Ibrutinib is an inhibitor of Bruton’s tyrosine kinase (BTK); however, the effect of Ibrutinib on intracellular MRSA survival was found to be independent of this pathway. Phosphoproteomics analysis of MRSA-infected cells identified candidate host kinases and phosphorylated proteins that may be responsible for the effects of Ibrutinib on MRSA. Intracellular MRSA activates n-wasp phosphorylation to promote the formation of actin comet-tails. n-wasp levels were increased in response to MRSA infection and decreased when infected cells were treated with Ibrutinib. However, the ratio of n-wasp/phospho-n-wasp was the same in untreated and Ibrutinib-treated infected cells, suggesting that Ibrutinib indirectly affects n-wasp. For this analysis, investigators relied on antibodies to n-wasp: NBP1-82512 and p-n-wasp: NB6001169, used in Western blot.
Ultimately, the ephrin receptor EphA2 was found to be the direct target of Ibrutinib, responsible for the decrease in intracellular MRSA in treated host cells. Importantly, EphA2 is involved in intracellular MRSA infections by other pathogens4.
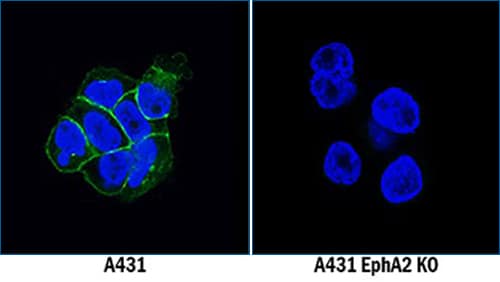
Genetic Strategies Validation. EphA2 was detected in immersion fixed A431 human epithelial carcinoma cell line but is not detected in EphA2 knockout (KO) A431 Human Cell Line cell line using Mouse Anti-Human EphA2 Monoclonal Antibody (Catalog # MAB3035) at 5 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 493-conjugated Anti-Mouse IgG Secondary Antibody (green; Catalog # NL009) and counterstained with DAPI (blue). Specific staining was localized to cell membranes.
The threat of increasing antibiotic resistance requires the identification of novel therapeutics to treat infectious disease. An appealing solution is repurposing drugs that are already known to be safe in humans. Additionally, in contrast to targeting bacterial viability, targeting host-cell processes decreases the risk of resistance in pathogens. Here, researchers have identified an FDA-approved drug, Ibrutinib, that decreases intracellular MRSA, a significant human pathogen that causes severe illness and is considered a serious threat due to its broad antibiotic resistance. Furthermore, Ibrutinib inhibits host factors that are known to be involved in other infections, both viral and bacterial, suggesting potential broad-spectrum capabilities of Ibrutinib as an antimicrobial. As MRSA internalization is also affected by ERK and JNK signaling inhibitors, this study opens the door to investigate other available kinase inhibitors in the treatment of S. aureus infections.
Explore potential kinase targets
 Beth Melson, MS
Beth Melson, MS
PhD Candidate
University of Virginia
Beth is working on her PhD studying virulence mechanisms of pathogenic E. coli.
References