 |
By Christina Towers, PhD.
Parkinson's disease is a debilitating neurodegenerative condition defined by the accumulation of alpha-synuclein-containing (alpha-SYN) intra-cytoplasmic inclusions, called Lewy bodies. The protein degradative processes of autophagy, and most specifically chaperone mediated autophagy and mitophagy, play an important protective role against this disease by targeting neurotoxins for lysosomal mediated degradation1. Alpha-SYN neurotoxins most commonly affect neurons, but they can also affect other supportive cell types in the brain including glial cells like astrocytes, although less is known about the consequences of alpha-SYN in other cell types2. Most, interestingly, alpha-SYN can transfer from one cell to the next propagating the disease. Cell transfer may occur via established mechanisms of cellular communication including secretory and exocytosis pathways as well as prion-like spreading3, however a recent publication by Rostami et al4 has identified tunneling nanotubes (TNTs) as a primary mechanism by which alpha-SYN can be shared between adjacent astrocytes in the brain.
These organelle and protein transporting highways were first described only 15 years ago5, but have been identified in many cell types in the brain and elsewhere including in cancer cells. But for the first time, TNTs have been identified in astrocytes where they play a role in two-way signaling. Specifically, the authors show that astrocytes treated with Cy3-labeled alpha-SYN oligomers cannot degrade the alpha-SYN oligomers despite interaction with lysosomal machinery, including Lamp-1. Instead, confocal microscopy and transmission electron microscopy (TEM) show that the buildup of alpha-SYN induces the formation of TNTs, which is dependent on actin filaments. Moreover, time-lapse microscopy shows alpha-SYN oligomers traveling through these TNTs between neighboring cells.
To further understand the signaling events that might regulate the formation of these nanotubes, the authors took a closer look at other organelle structures in alpha-SYN infected cells including, mitochondria, autophagosomes, and ER structures. They noted that alpha-SYN accumulation causes ER swelling, mitochondrial fragmentation, and inhibition of autophagic flux. In a handful of elegant experiments with unlabeled astrocytes treated with Cy3-labeled alpha-SYN oligomers and co-cultured with RFP labeled astrocytes, which were never exposed to Cy3- alpha-SYN, they show that alpha-SYN can transfer from an unlabeled cell to an RFP labeled cell. Furthermore, when similar experiments are performed with labeled mitochondria, results show that healthy cells transfer mitochondria to Cy3- alpha-SYN astrocytes, presumably in an attempt to rescue the fragmented mitochondrial phenotype induced by the neurotoxin.
|
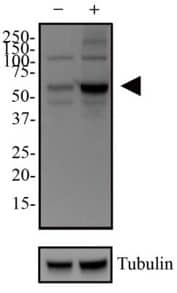
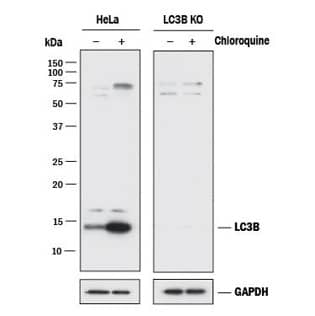
Method’s Highlight: To demonstrate that alpha-synuclein oligomers inhibit autophagy in astrocytes, investigators relied on two key antibodies from Novus Biologicals. (Rostami et al., J. Neuroscience, 2017; DOI: 10.1523/JNEUROSCI.0983-17.2017) |
|
|
|
These results are enlightening for Parkinson's disease, uncovering a potential therapeutic target, as the transfer of Cy3- alpha-SYN and the reciprocal transfer of healthy mitochondria could be restricted by pharmacological inhibition of actin polymerization with latrunculin B. But these results are also intriguing to a broader audience and suggest that ER stress, autophagy manipulation, or a change in mitochondrial dynamics could all regulate cell-cell signaling via tunneling microtubules across cell types and disease states.
Learn more about autophagy in neurodegeneration
 Christina Towers, PhD
Christina Towers, PhD
University of Colorado (AMC)
Dr. Towers studies the roles of autophagy, apoptosis and cell death in cancer.