Therapeutic proteins such as monoclonal antibodies (mAbs) can be subject to physical or chemical stress during the manufacturing process and supply chain. A common chemical degradation is the deamidation of the amino acid asparagine to aspartic acid or iso-aspartic acid. Modification of glutamine to glutamic acid is also possible, although this occurs at a slower rate. When deamidation occurs in the antigen binding region of the mAb, it can result in a loss of potency. This sequence liability may be defined as a critical quality attribute and represents a risk to the development of novel biologics and biosimilars alike. As such, it is important to have reliable protein characterization methods with sufficient sensitivity and selectivity to identify and quantify levels of deamidation.
The transformation of an amide side-chain, via a succinimide intermediate, to a carboxylic acid results in a change in the net charge of the protein. Thus charge-based separations, such as ion exchange chromatography or capillary isoelectric focussing, are commonly employed for the analytical characterization of deamidation.
Figure 2 shows a cation exchange chromatogram of mAb charge variants. In this method, the pH of the mobile phase was gradually increased over time, leading to separation of different protein forms according to their overall net charge in solution. Basic species are retained for longer than acidic species, therefore deamidated forms of the mAb are expected to elute before the main peak(s).
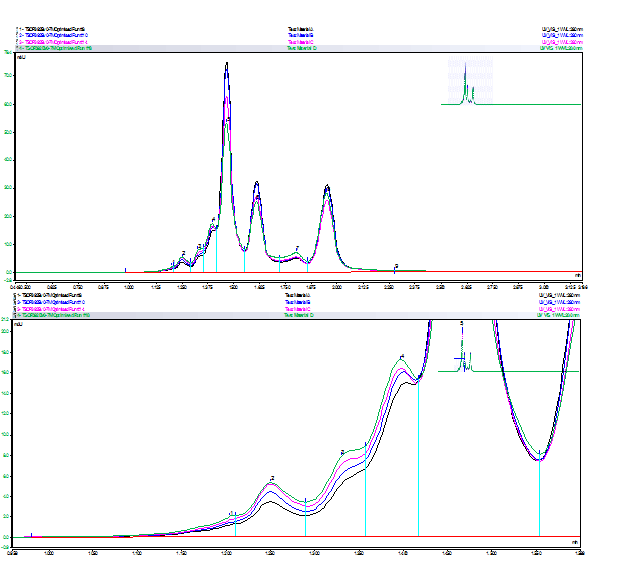
Figure 2 : Cation exchange chromatogram of mAb before/ after stress at 37 °C (above) and zoom of acidic region (below). Black: t = 0 h; blue: t= 18 h; magenta: t = 90 h; green: t = 1 week.
Interestingly, after less than 24 hours at 37 °C, there is a small but measurable increase in acidic variants at early retention times. These peaks are well-resolved and clearly distinguished from the main peaks. With increased stress (i.e. longer time at elevated temperature), there is a further increase in the relative abundance of acidic species, together with a decrease in the intensity of the main peaks. Although other modifications are also possible, it is likely that these acidic variants occur as a result of deamidation. A structure-function study would be required to determine impact of these changes on target binding and function.
As well as the variation in charge, deamidation also results in a mass increase of approximately 1 Da., whilst this difference is too small to measure on an intact mAb, a change in mass of this magnitude is readily detected at the peptide level by high resolution LC/MS instruments (Figure 3). Peptide mapping, where the protein is digested with a proteolytic enzyme prior to analysis, is therefore a useful technique for monitoring deamidation. This also allows the modification to be localized to a particular site.
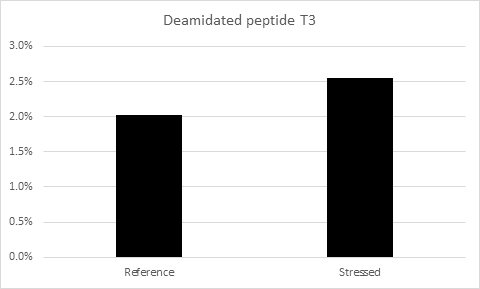
Figure 3 : Deconvolved mass spectra of an example unmodified peptide (above) and corresponding deamidated peptide (below), showing a mass shift of approximately 1 Da.
However, traditional sample preparation methods for peptide mapping employ overnight digestions at 37 °C and near-neutral pH. As shown above, these conditions are stressful enough to induce changes in the protein. Care should be taken to minimize modifications that occur as a result of the method, in order to distinguish them from real differences in the sample. Reducing the pH and/or digestion time may be necessary to minimize method-induced deamidation.
Using a peptide mapping approach, several sites of deamidation were identified for the mAb shown above. In each case, a higher level of deamidation was observed for the stressed sample compared to the reference standard (Figure 4). Together, ion exchange chromatography and peptide mapping represent powerful, orthogonal techniques for characterizing deamidation in therapeutic proteins.
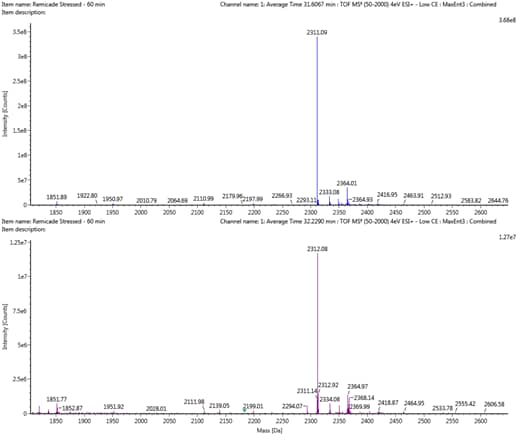
Figure 4 : Levels of deamidation detected for an example peptide before/after stress at 37 °C for 1 week.
By: Martin De Cecco, PhD from from Sartorius Stedim BioOutsource